You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
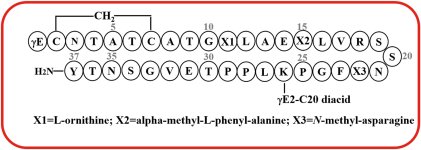
Eloralintide (LY3841136), a novel amylin receptor agonist for the treatment of obesity: From discovery to clinical proof of concept
- Thread starter leokitcat
- Start date
leokitcat
GLP-1 Apprentice
The structure/sequence is fully published here in this paper, so I expect research peptide availability will follow soon
Last edited:
leokitcat
GLP-1 Apprentice
Ha yeah, how many people on this forum see the early results from Eloralintide and wonder how long it will be before it shows up on the grey market?The structure/sequence is fully published here in this paper, so I expect research peptide availability will follow soon
I have some cagrilintide I bought early in my glp1 journey, thinking I might need it if my weight loss plateaus before reaching goal weight. Now it sounds like it has been obsoleted before I even needed it...
Thanks for posting this. I am really excited about Eloralintide. I am using Tirz and Reta in maintenance. I tried Cagri and it gave me a 24 hour a day headachache that went away when I stopped using it. I think Eloralintide and Reta would make the ultimate stack, and I'm guessing that maintenance would require a fairly low dose of both. I can't wait to start experimenting.The structure/sequence is fully published here in this paper, so I expect research peptide availability will follow soon
penewbie
GLP-1 Specialist
Cagri really helps me out, so Elora is probably going to be amazing. Can't come soon enough.
teabitten
Recently Joined
I will absolutely 100% switch my, uh, research subject over to Elora from Cagri as soon as it becomes available.


IshimaruKenta
GLP-1 Enthusiast
I'm curious how expensive this'll be. I know cagri can be pretty expensive, so this one will probably be more.
nonyabizznez
GLP-1 Specialist
Cagrilintide won't be obsolete. It's still a long-acting amylin that increases satiety and aids in weight loss. Aleve doesn't make make Tylenol obsolete. Jiffy doesn't make Skippy obsolete.Ha yeah, how many people on this forum see the early results from Eloralintide and wonder how long it will be before it shows up on the grey market?
I have some cagrilintide I bought early in my glp1 journey, thinking I might need it if my weight loss plateaus before reaching goal weight. Now it sounds like it has been obsoleted before I even needed it...
If there's no fibril/oligomer issue that might be another story. Elora sounds like a nice name for a queen oligomer in a Pixar movie though.
gulangaloid
GLP-1 Apprentice
cagri isn't very expensive considering how low the effective doses areI'm curious how expensive this'll be. I know cagri can be pretty expensive, so this one will probably be more.
leokitcat
GLP-1 Apprentice
Elora looks like will be stronger and have some fewer side effects. But cagri is strong at appetite control so you should still probably take advantage and use it as a combo with tirz / sema / reta if you need.Ha yeah, how many people on this forum see the early results from Eloralintide and wonder how long it will be before it shows up on the grey market?
I have some cagrilintide I bought early in my glp1 journey, thinking I might need it if my weight loss plateaus before reaching goal weight. Now it sounds like it has been obsoleted before I even needed it...
Trending Topics
Latest Posts
-
-
-
Joe Rogan on Peptide legislation: They're trying to keep like certain peptides from becoming legal
- Latest: exploitedworkerbee
Members Online
- foxgirl
- spacedogco
- Slogger
- m100568
- Bulldogg
- scout5
- qquag
- strixsir
- edumetzker
- New2ton
- Thatsmydoguk
- Nailedit
- Lolodu
- Peptide Bandit
- Momota
- c0c0_ly
- SimplyMe
- rkl123
- yeddie
- Holmesy1805
- Peptidesstacker
- shadyshades
- Macmac
- SwoleIsTheGoal
- Emjay752
- AProton3333
- Gorby2025
- throwmeaway
- tirzn00b
- JCPro
- Kingdaviddd
- Naphil
- CandyCap
- gerlswirl77
- glpbacon
- ftv10hb
- Walker Chemical
- Sassyqueen
- rp1986
- Harper
- wkndworrier
- mandible33
- Gietos
- Mounjamen
- alexispp
- princessofmilk
- Christini16
- waterdrinker
- Canuck54321
- tereaturquoise
Total: 542 (58 Members & 484 Guests)