penewbie
GLP-1 Specialist
There's been rumors of this for quite a while, specifically I first heard about this from Man On The Pen, that Lilly is running high dosage trials, being 20 and 25mg, but as of yet, I haven't seen any official confirmation. What we did have confirmation of is Lilly running "Investigational dosage" trials, but the mg amount was not specified.
Well I was fucking around googling shit, and I happened upon an Eli Lilly patent, wherein they completely lay out their trial step-by-step, and there it was, in black on white.

Guess they don't have a 25mg dose pen yet, they will actually have to jab twice with the low dosage pens.
Now, with that being said, hypothetically, why would I want to stack Reta, when I could just just increase my Tirz dosage to 25mg?
Oh, btw, that patent had another interesting tidbit, where they ran a trial looking at the degradation of Tirz over a period of 6 months, and the results sure did surprise me:
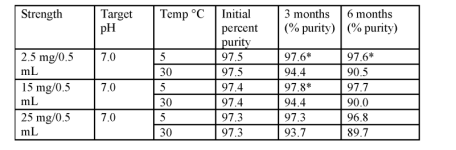
The liquid Tirzepatide, even at 30 degree Celsius for a period of 6 months, only lost a few percent purity. While at 5 degree Celsius, it lost barely anything at all. Granted, they do add some stabilizer to the mixtures, but still, if the liquid Tirz is this stable, then surely lyophilized Tirz is basically bullet proof.
Well I was fucking around googling shit, and I happened upon an Eli Lilly patent, wherein they completely lay out their trial step-by-step, and there it was, in black on white.

WO2024006662A1 - Tirzepatide compositions and use - Google Patents
A composition and methods of dosing such tirzepatide composition, comprising a tirzepatide, or a salt thereof at a concentration selected from the group consisting of 2.5 mg/mL, 40 mg/mL, and 50 mg/mL; NaCl; and dibasic sodium phosphate is provided.
patents.google.com
Guess they don't have a 25mg dose pen yet, they will actually have to jab twice with the low dosage pens.
Now, with that being said, hypothetically, why would I want to stack Reta, when I could just just increase my Tirz dosage to 25mg?
Oh, btw, that patent had another interesting tidbit, where they ran a trial looking at the degradation of Tirz over a period of 6 months, and the results sure did surprise me:

The liquid Tirzepatide, even at 30 degree Celsius for a period of 6 months, only lost a few percent purity. While at 5 degree Celsius, it lost barely anything at all. Granted, they do add some stabilizer to the mixtures, but still, if the liquid Tirz is this stable, then surely lyophilized Tirz is basically bullet proof.
