You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
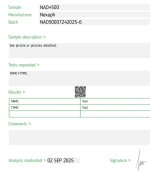
MIX sterility fail
- Thread starter fatty33
- Start date
geoguy78
GLP-1 Specialist
This is kind of what I was getting at in my comment on the other thread about people testing obsessively. Sterility testing is a prime example. We have immune systems, we cut and puncture ourselves with objects less sterile than these peps all the time. If you're worried about it, just buy a cheap filter and use good quality bacteriostatic water rather than spend hundreds on a test.Sterility fails are very very common. That's why most people don't test for them, why there are so many conversations about using Bac water and so many conversations about filtering.
IshimaruKenta
GLP-1 Enthusiast
Some princesses make such a big deal out of this. When ABC had a failed test, a few "ladies" told me to kill myself because I told them this wasn't a big deal.
Is Jano running a qualitative or quantitative ? I'm assuming the former since he just puts fail and I would assume it is faster/cheaper to run.Some princesses make such a big deal out of this. When ABC had a failed test, a few "ladies" told me to kill myself because I told them this wasn't a big deal.
Chucky
GLP-1 Apprentice
Wow, that's a tad extreme.Some princesses make such a big deal out of this. When ABC had a failed test, a few "ladies" told me to kill myself because I told them this wasn't a big deal.
I had questions about what was left in the remainder of the ~1% that isn't peptide on test results. Got this from Gemini and thought it was interesting:
Process-Related Impurities
These are non-peptide materials that remain from the reagents and solvents used during the synthesis and purification steps.
• Residual Solvents and Reagents: The final step of peptide synthesis often involves cleavage from the solid support using strong acids like Trifluoroacetic Acid (TFA). Some of this acid remains, and the peptide is often isolated as a TFA salt. Other solvents or scavengers (reagents used to prevent unwanted side reactions) can also remain.
• Salts and Buffers: The purification step, typically High-Performance Liquid Chromatography (HPLC), uses various buffers and salts. Trace amounts of these can remain in the final lyophilized (freeze-dried) powder.
• Elemental Impurities: Trace amounts of heavy metals or other elements that may have been present in the raw materials or from the reaction vessel can be present.
• Contaminating Peptides: In some rare cases, if multiple peptides are synthesized in the same facility, cross-contamination with a small amount of a completely different peptide can occur.
Process-Related Impurities
These are non-peptide materials that remain from the reagents and solvents used during the synthesis and purification steps.
• Residual Solvents and Reagents: The final step of peptide synthesis often involves cleavage from the solid support using strong acids like Trifluoroacetic Acid (TFA). Some of this acid remains, and the peptide is often isolated as a TFA salt. Other solvents or scavengers (reagents used to prevent unwanted side reactions) can also remain.
• Salts and Buffers: The purification step, typically High-Performance Liquid Chromatography (HPLC), uses various buffers and salts. Trace amounts of these can remain in the final lyophilized (freeze-dried) powder.
• Elemental Impurities: Trace amounts of heavy metals or other elements that may have been present in the raw materials or from the reaction vessel can be present.
• Contaminating Peptides: In some rare cases, if multiple peptides are synthesized in the same facility, cross-contamination with a small amount of a completely different peptide can occur.
tncc_rn
GLP-1 Enthusiast
I had questions about what was left in the remainder of the ~1% that isn't peptide on test results. Got this from Gemini and thought it was interesting:
Process-Related Impurities
These are non-peptide materials that remain from the reagents and solvents used during the synthesis and purification steps.
• Residual Solvents and Reagents: The final step of peptide synthesis often involves cleavage from the solid support using strong acids like Trifluoroacetic Acid (TFA). Some of this acid remains, and the peptide is often isolated as a TFA salt. Other solvents or scavengers (reagents used to prevent unwanted side reactions) can also remain.
• Salts and Buffers: The purification step, typically High-Performance Liquid Chromatography (HPLC), uses various buffers and salts. Trace amounts of these can remain in the final lyophilized (freeze-dried) powder.
• Elemental Impurities: Trace amounts of heavy metals or other elements that may have been present in the raw materials or from the reaction vessel can be present.
• Contaminating Peptides: In some rare cases, if multiple peptides are synthesized in the same facility, cross-contamination with a small amount of a completely different peptide can occur.
If ever people wondered if all these resellers are getting their supply from the same manufacturer, wonder no further.
If one seller has Triz that fails testing, guaranteed other resellers will have Triz in the same dose range fail the same tests. This is because most of these sellers get their wholesale products from the same manufacturer.
Probably can’t kill yourself with a failed vial though.Wow, that's a tad extreme.
Can you ask that AI for more detail? A list of those buffers and salts for solid phase synthesis would be nifty and useful.I had questions about what was left in the remainder of the ~1% that isn't peptide on test results. Got this from Gemini and thought it was interesting:
Process-Related Impurities
These are non-peptide materials that remain from the reagents and solvents used during the synthesis and purification steps.
• Residual Solvents and Reagents: The final step of peptide synthesis often involves cleavage from the solid support using strong acids like Trifluoroacetic Acid (TFA). Some of this acid remains, and the peptide is often isolated as a TFA salt. Other solvents or scavengers (reagents used to prevent unwanted side reactions) can also remain.
• Salts and Buffers: The purification step, typically High-Performance Liquid Chromatography (HPLC), uses various buffers and salts. Trace amounts of these can remain in the final lyophilized (freeze-dried) powder.
• Elemental Impurities: Trace amounts of heavy metals or other elements that may have been present in the raw materials or from the reaction vessel can be present.
• Contaminating Peptides: In some rare cases, if multiple peptides are synthesized in the same facility, cross-contamination with a small amount of a completely different peptide can occur.
Thanks I had no clueSterility fails are very very common. That's why most people don't test for them, why there are so many conversations about using Bac water and so many conversations about filtering.
Calm Logic
GLP-1 Specialist
Last edited:
IshimaruKenta
GLP-1 Enthusiast
Yeah, she also told me that I was choking on Allen's dick, called me a shill and a fat slob of all things. People are fucking ridiculous.Wow, that's a tad extreme.
Chucky
GLP-1 Apprentice
Sounds like one of those chicks you see on youtube crying that men don't ask her out on dates.Yeah, she also told me that I was choking on Allen's dick, called me a shill and a fat slob of all things. People are fucking ridiculous.
latviantower
GLP-1 Enthusiast
Yes. Critters in the pep that can be cultured and will reproduce in a Petri dish, or theoretically in your rat’s body. Unexpected chemicals present would be an issue of purity.Doesn't "sterility" specifically refer to viable microorganisms, rather than unexpected chemical components?
I’m assuming Jano tests for sterility by reconning the sample with sterile water, not BAC. But I’m not sure BAC would neutralize any viable critters anyway - that % of alcohol is pretty darn low. Meant to prevent future bacterial growth, not necessarily kill what’s already there.
Luckily, the human body has a relatively robust immune system. This is almost certainly a non-issue, though I wouldn't go looking to specifically buy these failed peptides. I am not sure I have ever put a peptide in my body that had a specific sterility test for it. I am still here.
You asked for it, so here it comes...Can you ask that AI for more detail? A list of those buffers and salts for solid phase synthesis would be nifty and useful.
Based on the process-related impurities outline you provided, specifically regarding the purification steps (HPLC), here are the specific examples of salts and buffers that are commonly left behind in peptide synthesis.
Common Residual Salts and Buffers
In the context of peptide synthesis, "salts" usually refer to the counter-ions attached to the peptide, while "buffers" refer to the agents used to maintain pH during purification.
1. Trifluoroacetate (TFA Salts)
* Source: As mentioned in your text, Trifluoroacetic Acid (CF_3COOH) is used for cleavage. However, it is also the most common ion-pairing agent added to the mobile phase in HPLC purification.
* Result: Because peptides are basic, they react with the acid to form a salt. Most research-grade peptides are delivered as a TFA salt, meaning the peptide carries a positive charge and is ionically bonded to a trifluoroacetate anion (CF_3COO^-).
2. Acetate (Acetate Salts)
* Source: Acetic acid (CH_3COOH) or Ammonium Acetate.
* Result: Since TFA can be toxic to cells in high concentrations, an additional salt exchange step is often performed to replace the TFA ion with an Acetate ion. Residual amounts of Ammonium Acetate or Acetic Acid may remain.
3. Hydrochloride (HCl Salts)
* Source: Hydrochloric Acid (HCl).
* Result: Used frequently for pharmaceutical-grade peptides because it is biologically common (similar to stomach acid). This leaves the peptide as a Hydrochloride salt (Cl^- counter-ion).
4. Volatile Buffers (HPLC Residuals)
During the lyophilization (freeze-drying) process, chemists prefer "volatile" buffers that evaporate along with the water. However, trace amounts can remain trapped in the powder lattice.
* Ammonium Bicarbonate: A common volatile buffer used when a basic pH is required.
* Formic Acid / Formate: Similar to acetic acid, used for pH control in LC-MS (Liquid Chromatography-Mass Spectrometry).
* Triethylamine (TEA): Sometimes used as an ion-pairing agent, though less common due to its strong odor and difficulty to remove.
5. Non-Volatile Salts (Contaminants)
These are less desirable because they do not evaporate during freeze-drying and result in high salt content in the final powder.
* Sodium Phosphate (Na_3PO_4)
* Sodium Chloride (NaCl)
* Potassium salts
Why do these remain?
As noted in your text's "Salts and Buffers" section, these materials are introduced during HPLC.
Understanding these residual elements is critical because they can significantly affect the stability, solubility, and even the biological activity of your peptide in downstream applications.
Edit: formattingNote: A peptide is rarely a neutral molecule; it is almost always a salt. If you have 1 mg of peptide powder, it might actually be 80% peptide and 20% residual salt/counter-ions and water.
Last edited:
Yes and no. “Manufacturers” make the raw peptide and “Finishers” lyophilize the pep. These are typically separate companies. There are more finishers than manufacturers. It’s possible that the same raw peptide from a manufacturer that is sold to 3 finishers fails testing from 2 finishers and passes testing from the third. It’s also possible that all 3 finishers receive bad product from the manufacturer.If ever people wondered if all these resellers are getting their supply from the same manufacturer, wonder no further.
If one seller has Triz that fails testing, guaranteed other resellers will have Triz in the same dose range fail the same tests. This is because most of these sellers get their wholesale products from the same manufacturer.
Yea, I know, a little hair splitting but that’s how the market is set up.
Varixdos
GLP-1 Novice
Meh. Anyone that's used gear just looks and shrugs at things like this.
There's likely to be more bacteria on the door knob to a public restroom.
There's likely to be more bacteria on the door knob to a public restroom.
100%Meh. Anyone that's used gear just looks and shrugs at things like this.
There's likely to be more bacteria on the door knob to a public restroom.
latviantower
GLP-1 Enthusiast
At the end of the day, I’ve got 7 kits of this batch of MIX T60 (replacing my original 6 kits that turned out to be 52/14 tirz/reta unintended blend). Vacuumed sealed with desiccant pack in the bottom of the chest freezer. When I work my way down to those (2027-2028?), I’ll just recon and filter and research away, most likely having forgotten about this thread.
Unexpected chemicals would be found in GCMS testing.Yes. Critters in the pep that can be cultured and will reproduce in a Petri dish, or theoretically in your rat’s body. Unexpected chemicals present would be an issue of purity.
I’m assuming Jano tests for sterility by reconning the sample with sterile water, not BAC. But I’m not sure BAC would neutralize any viable critters anyway - that % of alcohol is pretty darn low. Meant to prevent future bacterial growth, not necessarily kill what’s already there.
HPLC purity isn't testing for unexpected chemicals. It's focused on the band that represents what you're testing for, and the things that are just slightly out of range are basically degraded bits of the peptide.
You could have a vial that is majority contaminated with a cleaning agent and still get a 99% purity on hplc
are you ever going to experiment with the unintended blend?At the end of the day, I’ve got 7 kits of this batch of MIX T60 (replacing my original 6 kits that turned out to be 52/14 tirz/reta unintended blend). Vacuumed sealed with desiccant pack in the bottom of the chest freezer. When I work my way down to those (2027-2028?), I’ll just recon and filter and research away, most likely having forgotten about this thread.
latviantower
GLP-1 Enthusiast
Oh yeah I’ve been researching it weekly. Once my rat got over being surprised, I adjusted the dosing. It’s basically 4 parts tirz and 1 part reta. 10% overfill. 45 units vs 50 (30 mg/ml concentration). Appetite suppression is good. After being stalled for a month on straight tirz, this reignited some weight loss. Testing fasting glucose daily - no hypoglycemia. Good healthy little rat.are you ever going to experiment with the unintended blend?
Calm Logic
GLP-1 Specialist
You could resell it at a profit, haha. Could help at some point for transitioning to reta too.It’s basically 4 parts tirz and 1 part reta.
Kinda like qsc's tirz/Sema combo. I love that it's working out.Oh yeah I’ve been researching it weekly. Once my rat got over being surprised, I adjusted the dosing. It’s basically 4 parts tirz and 1 part reta. 10% overfill. 45 units vs 50 (30 mg/ml concentration). Appetite suppression is good. After being stalled for a month on straight tirz, this reignited some weight loss. Testing fasting glucose daily - no hypoglycemia. Good healthy little rat.