I'm glad to see some of my own thinking echoed here. The reliability of lot codes (or cap colors) could only be influenced by the original manufacturers in collaboration with distributors, and I just don't think they have any incentive (for security reasons). Even if they wanted to label, it would have to be with printed unique codes on the caps/bottles (though fluorescence labeling of the lyoph would be cool), and in my short time I've seen plenty of mistakes here which are so reputationally bad they must be screw-ups. Frankly, other than more testing/filtering I'm not sure the compounding pharms are that much better. I certainly had a batch from one I didn't trust (ended up alternating 2 sources for 4 weeks) never went back and tossed out jabs at $100 each.
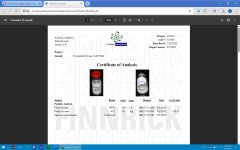
When group buys get different color caps that are supposedly to be the same batch, you have to laugh or cry (I guess it's better than getting the same color for different peps). I almost think that with the data available on fill%, purity, and cap colors from different vendors over time there's almost enough statistical data to figure how many sources there are and which distributors use which ones. Maybe that's part of what Finnrick is up to?