Hey,
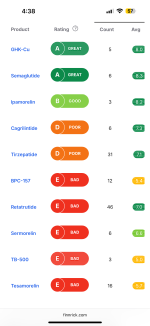
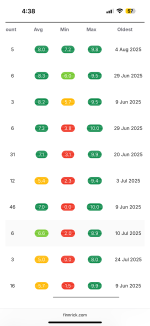
One of the reasons SSA was trusted is there has been EXTENSIVE testing on their product. I'd say their testing is on par with most of the bigger vendors- QSC back in the day, SRY, even Nexa. The COA's are often not posted here, since people tend to do group testing and do not want to share a test they funded to people who did not contribute. I've joined many of those tests myself; it's not uncommon for the rule to be results are not released unless something dangerous is found.
For your future reference, endotoxin testing has largely been found to be a waste of time and money. As far as I'm aware, an unsafe level has not ever been found.
Sterility is also largely a waste of time.
Mass and purity are usually done in the same test, so if you test for mass you will also receive a purity score. I'd consider doing some research into what purity means in the case of our research; it's largely a useless number used for marketing and to make us feel good. It only measures how much of the product similar to what we are testing for is actually the product. If they aren't testing for arsenic, they won't find the arsenic.
Finnrick is not how I'd suggest finding a vendor in the future, but I'm glad it's worked out for you! I would search for some of the interviews Peptide Test and Janoshik have done to better understand the science behind testing, and why not all labs are equal.