You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SS-31 + MOTs-C vs NAD+
- Thread starter Desieldogg
- Start date
ContainHer
GLP-1 Apprentice
Is anyone filtering their NAD+? I'd like to filter mine but wasn't sure if it can be done.
PAPoots
GLP-1 Enthusiast
You had me a freaky orange stuff.Somewhere in there someone should work in 5A1MQ. Mix in that freaky orange stuff for added energy and increased NAD+ through the NNMT inhibition.
PAPoots
GLP-1 Enthusiast
You might ask that in the Reta forum. For me, no. The higher heart rate, more frequent trips to the bathroom... according to my sleep tracker I get a lot less REM sleep. So rarely do i dream. I'm hoping long term my body adjusts, or when I get to my goal weight, I can back off enough to get better sleep.Do any of you notice having more dreams when taking RETA? I hope this doesn't last long because they are not always pleasant dreams, and I remember them too long.
AndyPanda
GLP-1 Specialist
Get yourself some Prazosin. It will stop your dreams.Do any of you notice having more dreams when taking RETA? I hope this doesn't last long because they are not always pleasant dreams, and I remember them too long.
I'm doing the same. I looked at cerebrolysin but I think I'm going to start with Epitalon for brain health first followed by Semax, both via IN. I read that Epitalon potentially helps with TBIs by restoring brain signal intensity. I've had a couple due to car accidents, none my fault. I read that Epitalon followed by Cerebrolysin acts synergistically. So, continuing Reta, followed by the mitochondrial protocol, followed by KLOW and then the brain health protocol, sequentially.74 year old male. My research is to follow the 14 week mitochondria protocol for molecular repair and enhancement. After that, my research will be 6 weeks on followed by 3 weeks off for a few cycles of GLOW. This will benefit cellular repair and enhancement. Molecular first followed by cellular.
Could you please please please link me to the pdf with the research documentation? I’m not finding it somehow.This is a 14 Week protocol. Weeks 13 and 14 are the same as Week 12..MOTS-c and NADS+. The PDF has links to files and research documentation to explain the reasoning behind this schedule. I started it Firday.
Does your pdf have the references to the research supporting the protocol? Would you mind sharing that with me please?Did you make it or did it originate here? I just had the PDF shared with me.
It has all that info. I attached it.Does your pdf have the references to the research supporting the protocol? Would you mind sharing that with me please?
Attachments
Ah, thank you so much! I see the attachment. ETA oooh very meaty and presented so thoroughly!It has all that info. I attached it.
I found the research quite compelling. I'll be starting it in about 10 days as written. As I posted above, some people did mention that these dosages might be a bit high for a younger researcher.Ah, thank you so much! I see the attachment. ETA oooh very meaty and presented so thoroughly!
Yeah there is so much good stuff all laid out! This is so very helpful,I found the research quite compelling. I'll be starting it in about 10 days as written. As I posted above, some people did mention that these dosages might be a bit high for a younger researcher.
I did see the rationale for lower doses, since it seems to work in the non-congenital population. And that new study uses lower doses also.
Are you just hoping for general increased vitality? (apologies and ok to just ignore question if too personal) Please let us know your experience!
I'm 67 so it's kind of a no-brainer. I've been taking NR orally for 10 years, so I think I'm prepped there. I'm just going to see if I can recapture some of the energy I used to have. My main concern for all of this research is aging gracefully and independently with all my facilities and mobility.Yeah there is so much good stuff all laid out! This is so very helpful,
I did see the rationale for lower doses, since it seems to work in the non-congenital population. And that new study uses lower doses also.
Are you just hoping for general increased vitality? (apologies and ok to just ignore question if too personal) Please let us know your experience!
That last line is so key and so fraught what with the rising incidence of dementia worldwide. I have family history and hisotry of injury also.I'm 67 so it's kind of a no-brainer. I've been taking NR orally for 10 years, so I think I'm prepped there. I'm just going to see if I can recapture some of the energy I used to have. My main concern for all of this research is aging gracefully and independently with all my facilities and mobility.
Well the brain exercise even sorting out and setting up this stuff should also help.
PAPoots
GLP-1 Enthusiast
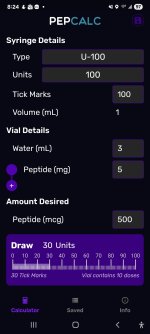
Your last line is key for me. After a few more months of research, I won't need pepcalc anymore. I'll be doing mg, mcg, ml, and unit calcs in my sleep.That last line is so key and so fraught what with the rising incidence of dementia worldwide. I have family history and hisotry of injury also.
Well the brain exercise even sorting out and setting up this stuff should also help.
You can then help scads of people online with their calculations… this is some amazing sort of person to person training in taking one’s health back.Your last line is key for me. After a few more months of research, I won't need pepcalc anymore. I'll be doing mg, mcg, ml, and unit calcs in my sleep.
PAPoots
GLP-1 Enthusiast
lessthanhalf
GLP-1 Enthusiast
Referring to the original post.
The problem with the protocol , and the article attatched, is the implication that these substances have been studied in humans, are safe, and the effects are known, and beneficial. Mostly this is not actually true. In general, the effects of the substances in humans individually are either not known at all, as for Mots c , or not well understood , or not very effective. Combined the effects are completely unknown, and have never been tested in animals or humans.
Many of the references are to something that is not a peer reviewed journal, the international peptide society, I tried to look it up to get a better idea of what it was but their website did not work. But mixing up genuine scientific references to animal studies with non scientific references is at best misleading.
SS-31 or Elamipretide has had human trials, so is known to be safe. Apart from treatment for Barth's syndrome, a genetic mitochondrial disorder, which was successful, the results of the human trials were generally disappointing, failed to meet their end points, and its use for more generalised purposes such as heart failure have been abandoned.
Mots c sounds amazing from animal studies, but has not been tested in humans. It is present naturally in the body so that gives it a bit of a safety factor, but the lack of any testing in humans means it effects in humans are currently completely unknown. There is definite interest from the drug companies in it and modifications to the peptide have been made and tested, mainly so it can be patented, and further research will happen.
There have been quite a few trials of various compounds to increase cellular NAD+, mainly NMN and N Riboside. They showed great promise in animal studies, and it is an important biological chemical, the question is - has it been proved that supplementation has beneficial effects in general or on specific disease states in humans? It has been studied enough to know that it is safe but effectiveness is much more questionable. There are lots of small studies so finding one that shows a benefit is easy, but in general unless enormous very expensive studies are done ( which is not going to happen unless it can be patented ) the easiest way to asses the research is a meta analysis that adds them all together and there are quite a few of them. For example - DOI: 10.1055/a-2382-6829
It's conclusions were pretty underwhelming
"Supplementation with NAD + precursors reduced plasma levels of total cholesterol and triglycerides in volunteers, but the intervention did not significantly affect the other outcomes analyzed *. Three of the included articles presented a high risk of bias. The quality of evidence varied between very low and low due to the risk of bias, imprecision, and indirectness. The number of participants in outcomes other than lipidemia is still generally tiny; therefore, more clinical trials evaluating these parameters will increase the quality of the evidence."
*outcomes tested were
Fasting blood glucose. Fasting blood insulin. Glycated hemoglobin.
HOMA index. Systolic blood pressure. Diastolic blood pressure. Body weight.
Cholesterolemia. Plasma LDL cholesterol levels. Plasma HDL cholesterol levels. Triglyceridemia.
This does not mean that these substances alone or together might not have benefits, they might but it is not known. My objection is that information presented in the protocol and the accompanying article very strongly imply that the science of this recommendation is established fact, it is not. I would guess no reputable clinicians ( which does not include celebrity doctors or similar who sadly seem more interested in making money than providing sound advice ) would recommend mots c or ss-31 on the basis of current available evidence. NAD+ therapies are almost certainly safe, but there is a serious lack of evidence that they work in humans, except for low quality evidence they lower lipid levels and statins are much more effective at lowering blood lipids.
The problem with the protocol , and the article attatched, is the implication that these substances have been studied in humans, are safe, and the effects are known, and beneficial. Mostly this is not actually true. In general, the effects of the substances in humans individually are either not known at all, as for Mots c , or not well understood , or not very effective. Combined the effects are completely unknown, and have never been tested in animals or humans.
Many of the references are to something that is not a peer reviewed journal, the international peptide society, I tried to look it up to get a better idea of what it was but their website did not work. But mixing up genuine scientific references to animal studies with non scientific references is at best misleading.
SS-31 or Elamipretide has had human trials, so is known to be safe. Apart from treatment for Barth's syndrome, a genetic mitochondrial disorder, which was successful, the results of the human trials were generally disappointing, failed to meet their end points, and its use for more generalised purposes such as heart failure have been abandoned.
Mots c sounds amazing from animal studies, but has not been tested in humans. It is present naturally in the body so that gives it a bit of a safety factor, but the lack of any testing in humans means it effects in humans are currently completely unknown. There is definite interest from the drug companies in it and modifications to the peptide have been made and tested, mainly so it can be patented, and further research will happen.
There have been quite a few trials of various compounds to increase cellular NAD+, mainly NMN and N Riboside. They showed great promise in animal studies, and it is an important biological chemical, the question is - has it been proved that supplementation has beneficial effects in general or on specific disease states in humans? It has been studied enough to know that it is safe but effectiveness is much more questionable. There are lots of small studies so finding one that shows a benefit is easy, but in general unless enormous very expensive studies are done ( which is not going to happen unless it can be patented ) the easiest way to asses the research is a meta analysis that adds them all together and there are quite a few of them. For example - DOI: 10.1055/a-2382-6829
It's conclusions were pretty underwhelming
"Supplementation with NAD + precursors reduced plasma levels of total cholesterol and triglycerides in volunteers, but the intervention did not significantly affect the other outcomes analyzed *. Three of the included articles presented a high risk of bias. The quality of evidence varied between very low and low due to the risk of bias, imprecision, and indirectness. The number of participants in outcomes other than lipidemia is still generally tiny; therefore, more clinical trials evaluating these parameters will increase the quality of the evidence."
*outcomes tested were
Fasting blood glucose. Fasting blood insulin. Glycated hemoglobin.
HOMA index. Systolic blood pressure. Diastolic blood pressure. Body weight.
Cholesterolemia. Plasma LDL cholesterol levels. Plasma HDL cholesterol levels. Triglyceridemia.
This does not mean that these substances alone or together might not have benefits, they might but it is not known. My objection is that information presented in the protocol and the accompanying article very strongly imply that the science of this recommendation is established fact, it is not. I would guess no reputable clinicians ( which does not include celebrity doctors or similar who sadly seem more interested in making money than providing sound advice ) would recommend mots c or ss-31 on the basis of current available evidence. NAD+ therapies are almost certainly safe, but there is a serious lack of evidence that they work in humans, except for low quality evidence they lower lipid levels and statins are much more effective at lowering blood lipids.
Calm Logic
GLP-1 Specialist
Except for a few newbies, I don't think people assume such protocols are gospel,. More like a shot in the dark, often trying to also balance cost with potential effectiveness.
For SS-31, one could argue for 40 mg a day instead of 4 mg, but who can afford that?
Regarding lab results, there is always more that can be tested. Like telomere testing, but that is expensive too. Not to mention heart imaging, nerve conduction tests, brain tests, etc.
But to echo an earlier comment, I see it as more like insurance -- you may not need it and they may not pay the claim, but you don't want to be without it either.
For SS-31, one could argue for 40 mg a day instead of 4 mg, but who can afford that?
Regarding lab results, there is always more that can be tested. Like telomere testing, but that is expensive too. Not to mention heart imaging, nerve conduction tests, brain tests, etc.
But to echo an earlier comment, I see it as more like insurance -- you may not need it and they may not pay the claim, but you don't want to be without it either.
Last edited:
Calm Logic
GLP-1 Specialist
Seems like a great plot for a movie. But seriously, it is interesting that the vividness of dreams is often not a good thing, especially when it is every night. That's one reason I didn't like Prozac.Get yourself some Prazosin. It will stop your dreams.
Mr. Blonde
GLP-1 Enthusiast
I slam Hypnocil. It will get rid of your nightmares even if you live on Elm Street.Get yourself some Prazosin. It will stop your dreams.
Get yourself some Prazosin. It will stop your dreams.
AndyPanda
GLP-1 Specialist
The VA doesn’t prescribe that. LolI slam Hypnocil. It will get rid of your nightmares even if you live on Elm Street.
PAPoots
GLP-1 Enthusiast
You would think the VA would prescribe that more than anyone.The VA doesn’t prescribe that. Lol
I know taking l-theanine before bed will make you have nightmares, or taking large doses of melatonin.
AndyPanda
GLP-1 Specialist
Prazosin is probably just cheaper. It works great for nightmares though. Just make you feel autonomous. You go to sleep, then you wake up. Nothing in between.You would think the VA would prescribe that more than anyone.
I know taking l-theanine before bed will make you have nightmares, or taking large doses of melatonin.
My #1 favorite drug of all time!!! My doc won’t prescribe it for me anymore as it was a crutch for poor sleep habits but I’ve kept a bottle for “emergencies” such as cross-country drives and having to pull a hat trick at work.My doctor gave me an Rx for modafinil. It was amazing.
miss-sanne
GLP-1 Enthusiast
I have NAD, but it turned to gel... Must make a new vial soon.
But I started Mots daily, it's amazing on it's own.
I can train way harder than usual, and I don't crash at 5 pm after work.
I didn't do the protocol though, because I don't have any SS-31.
But I started Mots daily, it's amazing on it's own.
I can train way harder than usual, and I don't crash at 5 pm after work.
I didn't do the protocol though, because I don't have any SS-31.
When do you pin the Mots relative to training? I’m trying to get this worked out so I can leverage that benefit.But I started Mots daily, it's amazing on it's own.
I can train way harder than usual, and I don't crash at 5 pm after work.
miss-sanne
GLP-1 Enthusiast
I do it 1 hour before training.When do you pin the Mots relative to training? I’m trying to get this worked out so I can leverage that benefit.
PAPoots
GLP-1 Enthusiast
How long does NAD last before turning to gel? Is this common. My vial is a dark orange, so it would be hard to tell, unless it really got thickI have NAD, but it turned to gel... Must make a new vial soon.
But I started Mots daily, it's amazing on it's own.
I can train way harder than usual, and I don't crash at 5 pm after work.
I didn't do the protocol though, because I don't have any SS-31.
noggin
GLP-1 Apprentice
I think this is pretty important and lot of people here either want to ignore it or glance by it without fully understanding it. I'm in no position to judge others for researching what they want but it's also important to be cognizant of the realities of the data behind these substances or lack thereof. I was under the impression MOTS-c had more human testing so this is an eye opener. I was about to start researching it but I might just not try it or start with a very low dose. Although the fact it's naturally occurring in the body (and a peptide, not a small molecule), and the promising 1a/1b human safety trials for the MOTS-c analogue CB4211 makes me feel better about researching.Referring to the original post.
The problem with the protocol , and the article attatched, is the implication that these substances have been studied in humans, are safe, and the effects are known, and beneficial. Mostly this is not actually true. In general, the effects of the substances in humans individually are either not known at all, as for Mots c , or not well understood , or not very effective. Combined the effects are completely unknown, and have never been tested in animals or humans.
Many of the references are to something that is not a peer reviewed journal, the international peptide society, I tried to look it up to get a better idea of what it was but their website did not work. But mixing up genuine scientific references to animal studies with non scientific references is at best misleading.
SS-31 or Elamipretide has had human trials, so is known to be safe. Apart from treatment for Barth's syndrome, a genetic mitochondrial disorder, which was successful, the results of the human trials were generally disappointing, failed to meet their end points, and its use for more generalised purposes such as heart failure have been abandoned.
Mots c sounds amazing from animal studies, but has not been tested in humans. It is present naturally in the body so that gives it a bit of a safety factor, but the lack of any testing in humans means it effects in humans are currently completely unknown. There is definite interest from the drug companies in it and modifications to the peptide have been made and tested, mainly so it can be patented, and further research will happen.
There have been quite a few trials of various compounds to increase cellular NAD+, mainly NMN and N Riboside. They showed great promise in animal studies, and it is an important biological chemical, the question is - has it been proved that supplementation has beneficial effects in general or on specific disease states in humans? It has been studied enough to know that it is safe but effectiveness is much more questionable. There are lots of small studies so finding one that shows a benefit is easy, but in general unless enormous very expensive studies are done ( which is not going to happen unless it can be patented ) the easiest way to asses the research is a meta analysis that adds them all together and there are quite a few of them. For example - DOI: 10.1055/a-2382-6829
It's conclusions were pretty underwhelming
"Supplementation with NAD + precursors reduced plasma levels of total cholesterol and triglycerides in volunteers, but the intervention did not significantly affect the other outcomes analyzed *. Three of the included articles presented a high risk of bias. The quality of evidence varied between very low and low due to the risk of bias, imprecision, and indirectness. The number of participants in outcomes other than lipidemia is still generally tiny; therefore, more clinical trials evaluating these parameters will increase the quality of the evidence."
*outcomes tested were
Fasting blood glucose. Fasting blood insulin. Glycated hemoglobin.
HOMA index. Systolic blood pressure. Diastolic blood pressure. Body weight.
Cholesterolemia. Plasma LDL cholesterol levels. Plasma HDL cholesterol levels. Triglyceridemia.
This does not mean that these substances alone or together might not have benefits, they might but it is not known. My objection is that information presented in the protocol and the accompanying article very strongly imply that the science of this recommendation is established fact, it is not. I would guess no reputable clinicians ( which does not include celebrity doctors or similar who sadly seem more interested in making money than providing sound advice ) would recommend mots c or ss-31 on the basis of current available evidence. NAD+ therapies are almost certainly safe, but there is a serious lack of evidence that they work in humans, except for low quality evidence they lower lipid levels and statins are much more effective at lowering blood lipids.
Trending Topics
Latest Posts
-
Numbness is ring, middle and index fingers. Is a peptide the cause.
- Latest: Peotidethrowaway
-
-
-
After a couple of delays, I’m finally starting my first week of tirz tomorrow.
- Latest: Need_to_shrink
Members Online
- Fam66
- Staticx
- LightAsAFeather
- KchTe
- swimmer
- Sparky757
- ibembhy
- Azuresands
- Muskott
- AlexSilver
- riposte
- RFlores87
- fasting4life
- leXkurb
- LIBERO
- IwaraShoa
- 390120
- MrHart
- Thedarkesttimeline
- woundcarping
- ostrichsak
- KhrisDaThird
- Othed2121
- ghreid
- alan wen
- Lucky206
- Bunny
- HereForThePeptides
- ApexBrew
- yml1966
- Eazy99
- fatbegone
- Eight90
- texas_girl01
- jw717us
- Bailliechowski
- latviantower
- crispynights
- Cassan
- Thedooman445
- Frankbooth
- V6R3W
- Dos-Dox
- Peotidethrowaway
- CWJ1888
- AllTheBacon
- NEKVT
- dndgeek
- yeddie
- DustAndDignity
Total: 314 (67 Members & 247 Guests)