I bought the T60 in early August (as I mentioned in the first few pages of this thread). I injected 4mg on Saturday (3 days ago). There was no stinging. It does feel like The product is over weight. I can feel that I'm on a slightly higher dose than usual, but that’s fine. I haven’t experienced any side effects.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
AmoPure Emerges From AmoPeptide Tirzwreck UPDATED*
- Thread starter dionysos
- Start date
Lankiegirl
Recently Joined
I just pinned my RS with L60mg. I was in the test grp for the August batch . So the vial did come as >99 purity. Waiting on sterility.
Also reconstituted and filtered using proper protocol that Peptest mentioned. No stinging and no burning. I get stinging and burning more with CT since I started months ago vs this.
Also reconstituted and filtered using proper protocol that Peptest mentioned. No stinging and no burning. I get stinging and burning more with CT since I started months ago vs this.
Received my order last week, 9/25. 30mg with blue tops. Most pucks broken. Straight to freezer.
Reconstituted with hospira bac, 2ml (2025 exp date bac water). Vacuum sealed, confirmed during reconstitution. Noticed no gel-ing like others have mentioned. Perfectly clear, stored in fridge overnight.
Injected rs today with 25 units after leaving out for ~30min.
Zero reported stinging or burning. Reportedly felt exactly the same as previous compounding injections.
Reconstituted with hospira bac, 2ml (2025 exp date bac water). Vacuum sealed, confirmed during reconstitution. Noticed no gel-ing like others have mentioned. Perfectly clear, stored in fridge overnight.
Injected rs today with 25 units after leaving out for ~30min.
Zero reported stinging or burning. Reportedly felt exactly the same as previous compounding injections.
PeptideJedi
GLP-1 Apprentice
So you take 100 UI each dose ?FWIW I had stinging from my red rocks version of Tirz at start as well. I now fill my syringe with dose, leave at room temp for 30min, then inject... and no more stinging.
Hello!Received my order last week, 9/25. 30mg with blue tops. Most pucks broken. Straight to freezer.
Reconstituted with hospira bac, 2ml (2025 exp date bac water). Vacuum sealed, confirmed during reconstitution. Noticed no gel-ing like others have mentioned. Perfectly clear, stored in fridge overnight.
Injected rs today with 25 units after leaving out for ~30min.
Zero reported stinging or burning. Reportedly felt exactly the same as previous compounding injections.
So I just got my first order from Amo, L15 😊
I am a newbie…is it normal for the product to be in rocks ?
I put my vials in the freezer & once my filter arrives I will reconstitute my first vial. I read about the possibility of stinging etc, so I will definitely report back! I am sooo thankful for this forum, especially in these times!! 😊
Hello!
So I just got my first order from Amo, L15 😊
I am a newbie…is it normal for the product to be in rocks ?
I put my vials in the freezer & once my filter arrives I will reconstitute my first vial. I read about the possibility of stinging etc, so I will definitely report back! I am sooo thankful for this forum, especially in these times!! 😊
If by rocks, you mean pucks of the dry powder (Amos seem to always break apart in shipping), then yes, that's normal.
PeptideJedi
GLP-1 Apprentice
That's normal for Amo since they are using a different filler it does not stay together when shipped..Hello!
So I just got my first order from Amo, L15 😊
I am a newbie…is it normal for the product to be in rocks ?
I put my vials in the freezer & once my filter arrives I will reconstitute my first vial. I read about the possibility of stinging etc, so I will definitely report back! I am sooo thankful for this forum, especially in these times!! 😊
Oh !! Is that why they are called pucks? I am kind of a visual learner so pucks is making more sense now 😂😂That's normal for Amo since they are using a different filler it does not stay together when shipped..
😂 yes, thank you! Pucks is making more sense now 😊If by rocks, you mean pucks of the dry powder (Amos seem to always break apart in shipping), then yes, that's normal.
PeptideJedi
GLP-1 Apprentice
They add filler usually mannitol to the peptide ..then sterile water and basically freeze dry all moisture out and yes it turns into a puck at bottom of vial ..but since Amo is using a different filler it doesn't stay compressed into the puck as well...the filler is lighter.😂 yes, thank you! Pucks is making more sense now 😊
Ahh! Makes sense. Thank you so much for answering this, I sincerely appreciate it! 😊They add filler usually mannitol to the peptide ..then sterile water and basically freeze dry all moisture out and yes it turns into a puck at bottom of vial ..but since Amo is using a different filler it doesn't stay compressed into the puck as well...the filler is lighter.
Vacation4us
GLP-1 Enthusiast
Just received my L60 today from Texas, arrived in 5 days. Will reconstitute today and use first dose Monday. My BAC water is Hospira ( professional grade, 😍 got it from work) being a nurse has perks.
I am not concerned about the burn but I will report back if it happens. Previously only been on Big Pharma. It hurts sometimes, I bruise sometimes, and sometimes I don’t even feel it. I also give good shot, and have zero fear of needles.
I am not concerned about the burn but I will report back if it happens. Previously only been on Big Pharma. It hurts sometimes, I bruise sometimes, and sometimes I don’t even feel it. I also give good shot, and have zero fear of needles.
nonyabizznez
GLP-1 Specialist
They are also called "cakes"... Amopure is more of a cake...a dry crumbly cake than a puck.Ahh! Makes sense. Thank you so much for answering this, I sincerely appreciate it! 😊
Dustycotton
GLP-1 Apprentice
Not sure if anyone still cares to see pH results of Amopure, but I just filled my first vial of L60 today that was shipped on 8/28/24 from China. Intact puck, completely dissolved to a clear liquid. New Hospira was used, exp. Nov 2025.
I was still finishing my last compounded vial from Red Rock, so I also pH tested that for comparison.
After this vial of Amo, I'm going to use actual Zep that I still have, and will test that as well. I am also keeping notes on each one for injection site issues, if any, and my own assessment of how well each works for me. All three are being taken at 12.5mg dose split at 3.5 days each week.
Note: I've never had an ISR or have felt any pain from any injection since starting tirz Dec 2023; either with the Zep pens or RR compounded. I don't take Amo until Monday evening, so I'll report back if I have any ISR or pain with that one. I filled the Amo 2 days before I need it to be similar to the RR vials I've been using, which are filled about 3-4 days before I receive them.
Strengths
Red Rock 20mg/ml
Amopure 24mg/ml
The RR pH is pretty close to 6 and the Amo is a bit closer to 7. But it seems both are between 6 and 7.
Red Rock
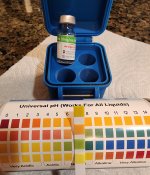
Amopure L60


I was still finishing my last compounded vial from Red Rock, so I also pH tested that for comparison.
After this vial of Amo, I'm going to use actual Zep that I still have, and will test that as well. I am also keeping notes on each one for injection site issues, if any, and my own assessment of how well each works for me. All three are being taken at 12.5mg dose split at 3.5 days each week.
Note: I've never had an ISR or have felt any pain from any injection since starting tirz Dec 2023; either with the Zep pens or RR compounded. I don't take Amo until Monday evening, so I'll report back if I have any ISR or pain with that one. I filled the Amo 2 days before I need it to be similar to the RR vials I've been using, which are filled about 3-4 days before I receive them.
Strengths
Red Rock 20mg/ml
Amopure 24mg/ml
The RR pH is pretty close to 6 and the Amo is a bit closer to 7. But it seems both are between 6 and 7.
Red Rock

Amopure L60


Dustycotton
GLP-1 Apprentice
Not sure if anyone still cares to see pH results of Amopure, but I just filled my first vial of L60 today that was shipped on 8/28/24 from China. Intact puck, completely dissolved to a clear liquid. New Hospira was used, exp. Nov 2025.
I was still finishing my last compounded vial from Red Rock, so I also pH tested that for comparison.
After this vial of Amo, I'm going to use actual Zep that I still have, and will test that as well. I am also keeping notes on each one for injection site issues, if any, and my own assessment of how well each works for me. All three are being taken at 12.5mg dose split at 3.5 days each week.
Note: I've never had an ISR or have felt any pain from any injection since starting tirz Dec 2023; either with the Zep pens or RR compounded. I don't take Amo until Monday evening, so I'll report back if I have any ISR or pain with that one. I filled the Amo 2 days before I need it to be similar to the RR vials I've been using, which are filled about 3-4 days before I receive them.
Strengths
Red Rock 20mg/ml
Amopure 24mg/ml
The RR pH is pretty close to 6 and the Amo is a bit closer to 7. But it seems both are between 6 and 7.
Update After Injection L60
I injected about 30 min ago from the L60 kit I purchased on 8/28/24 and felt absolutely nothing; same as every injection I've ever had from Red Rock vials. I did recon the vial on Saturday, so it sat for about two days in the fridge. I followed the same exact procedure as I've done with my Red Rock vials and used the same syringe brand/type. Vial was 24mg/ml strength and I injected 26 units (half my weekly dose).
Injection method that I've been using since starting compounded in March:
1. Remove vial from refrigerator.
2. Roll vial on flat surface gently 5 times to mix contents.
3. Alcohol wipe top of vial. Let air dry.
4. Pull back U100 syringe to desired units. Inject vial with air from syringe. Invert vial and draw to desired units. Recap syringe.
5. Hold filled syringe in closed hand for 5 minutes to warm up contents.
6. Fresh alcohol wipe on skin. Let air dry.
7. Pinch skin on abdomen and inject at a 90 degree angle.
SmokeySquid
GLP-1 Enthusiast
This brings up a question that I have had for a while now but do the peps "settle" for lack of a better word in the reconstituted solution? I have seen people mention this occasionally but haven't seen too many people actually discuss it. I always assumed that they were completely emulsified.2. Roll vial on flat surface gently 5 times to mix contents.
Dustycotton
GLP-1 Apprentice
No idea, could do absolutely nothing at all. Since it doesn't hurt anything to do this, and I've done it since starting compounded, it's just become routine for me. Not a very scientific reason, I know. It is something I've read as recommended to do before each does from multidose vaccine vials, but I've never researched the why behind this recommendation.This brings up a question that I have had for a while now but do the peps "settle" for lack of a better word in the reconstituted solution? I have seen people mention this occasionally but haven't seen too many people actually discuss it. I always assumed that they were completely emulsified.
Trending Topics
Latest Posts
-
-
PPHK 🎀 Welcome to Peptide Pole HK- Best Price & Quality
- Latest: Ella HKfatcat
-
-
Members Online
- Christini16
- Ted R
- StevieWonder123
- Hunni
- brdlphil
- Katkin
- OregonSunshine
- Gr33dyOctopus
- finnputz
- ObvCheat
- woondoo
- DazDillinger
- Purplepansy
- Manmountain
- betrayedlemon
- Ben2008
- FRMN01
- olivia ava
- coustang
- Luis1917dam
- VonHectic
- winiki
- Mr. Blonde
- qazjude
- CMA Pooky
- 10:22
- Justin Anon
- Brookie
- MangoritaNoPickle
- AProton3333
- Twowheelr2
- WeezinDaJuice
- gusterbuster
- klaudioz
- Rolltide61
- TRS123
- Pats-cats
- Honey18
- Rico suave
- CFJS42
- j88xx
- Sparky757
- 15stoneguy
- redbullwings
- MaxieChi
- chigirl
- Mannyn187
- UglyDingo
- kellywu463
- tjm134
Total: 267 (63 Members & 204 Guests)
