secretweapon
GLP-1 Apprentice
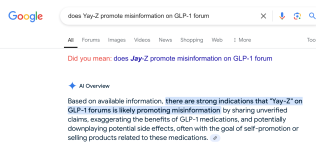
I sure thought that was strange. I took myself out of the equation by making it all about direct Novo quotes and people still want to shoot the messenger.Really wish we'd spend more time focused on what the OP has written and backed up with scientific evidence, rather than the OP himself.
Because this shit is pretty scary, I know we're used to all sorts of peptides of unknown benefit or harm, but here it seems we have a peptide with a likely known harm.