You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Orforglipron: The next big thing?
- Thread starter Derby
- Start date
Calm Logic
GLP-1 Specialist
I doubt there are such studies yet, but it should be fine.
Obesity specialists have been adding metformin occasionally for some people already on a GLP, like when someone on sema or tirz wants a boost but can't raise the dose yet due to the dosing schedule.
Obesity specialists have been adding metformin occasionally for some people already on a GLP, like when someone on sema or tirz wants a boost but can't raise the dose yet due to the dosing schedule.
Last edited:
Calm Logic
GLP-1 Specialist
By the way, brand-name Jardiance is available from India.
Personally, I don't see the need for having Jardiance or metformin along with a GLP, since a GLP is much stronger. (My internist wanted me to increase the dose for tirz even weekly, if necessary.)
OTOH, metformin increases the body's natural production of GLP-1. So it may be good while on a strict dosing schedule. Like people tend to feel hungrier on day five of a weekly dose of tirz.
Personally, I don't see the need for having Jardiance or metformin along with a GLP, since a GLP is much stronger. (My internist wanted me to increase the dose for tirz even weekly, if necessary.)
OTOH, metformin increases the body's natural production of GLP-1. So it may be good while on a strict dosing schedule. Like people tend to feel hungrier on day five of a weekly dose of tirz.
Last edited:
byefatlicia
GLP-1 Enthusiast
Have you seen anything on skyrizy?By the way, brand-name Jardiance is available from India. But I personally don't see the need for having Jardiance or metformin along with a GLP, since a GLP is much stronger.
Calm Logic
GLP-1 Specialist
Not really. The one reputuble-looking seller on IndiaMart sells it for over $1,000 USD, which is still cheap compared to the price at GoodRx. And according to Google Gemini, it is still under a patent in India.Have you seen anything on skyrizy?
byefatlicia
GLP-1 Enthusiast
Okay thanks🙂 the brand name goes for 19k :0Not really. The one reputuble-looking seller on IndiaMart sells it for over $1,000.
Calm Logic
GLP-1 Specialist
Yeah, I just noticed that and included the link to GoodRx. Insane.
Calm Logic
GLP-1 Specialist
Have any of the studies covered how Orforglipron interacts with metformin. I am still on metformin and don't want to quit when I start researching this.
From an article on polypharmacy:

Why Do GLP-1 Drugs Stop Working, and What to Do About It?
Everybody has a set point, and every weight loss intervention eventually leads to a plateau, so it helps to have a variety of tools to support patients.
"You don't see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They're on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They're usually on three, four, five drugs, similar to what we would see with resistant hypertension."
Last edited:
Calm Logic
GLP-1 Specialist
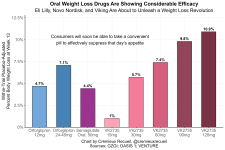
This is only true if 10% weight loss is considered "considerable " efficacy, I guess
Calm Logic
GLP-1 Specialist
Yes, to get good efficacy, one would ideally switch from orfo to tirz (or reta) at some point. At 12 weeks, orfo is almost similar to tirz in effect. As time goes on, the average differences become painfully obvious, according to the studies. This is similar in idea to switching from sema to tirz.
Last edited:
Thank you for sharing!!From an article on polypharmacy:

Why Do GLP-1 Drugs Stop Working, and What to Do About It?
Everybody has a set point, and every weight loss intervention eventually leads to a plateau, so it helps to have a variety of tools to support patients.www.medscape.com
"You don't see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They're on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They're usually on three, four, five drugs, similar to what we would see with resistant hypertension."
Calm Logic
GLP-1 Specialist
Gemini puts orfo in the same category as sema for effectiveness:
FINAL RANKING OF MEDICATIONS BY WEIGHT LOSS EFFECT (Highest to Lowest)
Rank Medication(s) Effectiveness Profile Typical Weight Loss (1 Year) 1. Setmelanotide (Imcivree) Very High (Specific Genetic Use) 12.5% - 25.6% (Only for specific rare genetic conditions) 2. Retatrutide (Investigational) Very High / Investigational 17% - 24.2% of initial body weight 3. Tirzepatide (Zepbound/Mounjaro) Very High 15% - 22.5% of initial body weight 4. Semaglutide (Wegovy/Ozempic) High 10% - 15% of initial body weight 5. Orforglipron (Investigational) High (Oral) 9.2% - 12.4% of initial body weight 6. Phentermine-Topiramate (Qsymia) High/Moderate 7% - 11% of initial body weight 7. Liraglutide (Saxenda) Moderate ~8% of initial body weight 8. Naltrexone/Bupropion (Contrave) Moderate 5% - 10% of initial body weight 9. Phentermine Moderate (Short-Term Use) 3% - 10% of initial body weight 10. Metformin & Jardiance (Combination) Modest 3% - 6% of initial body weight[/b] 11. Orlistat (Xenical/Alli) Modest ~3% - 4% greater than diet alone 12. Metformin Modest 2% - 5% of initial body weight 13. Jardiance (Empagliflozin) Modest 2% - 3% of initial body weight 14. Low-Dose Naltrexone (LDN) Minimal/Variable < 5% (Used alone, off-label)
SUMMARY OF DRUG CLASSES
- Very High Efficacy (Ranks 1-5): Primarily hormone agonists (GLP-1/GIP/Melanocortin system) that target the core biological mechanisms of appetite and metabolism.
- Moderate Efficacy (Ranks 6-9): Combination therapies and stimulants that use various mechanisms (appetite suppression, reward pathways) to achieve clinically significant, but generally lower, total weight loss.
- Modest Efficacy (Ranks 10-14): Older prescription drugs (Metformin, Jardiance) whose weight loss is a secondary effect, or fat-absorption blockers (Orlistat) and off-label single agents (LDN).
Last edited:
A pill form of GLP1 and GIP agonist would be revolutionary. I don’t know if a pill form of glucagon will be possible though, so oral tirzepatide would be the first goal. Oral retatrutide would be the dream.
Not a glucagon receptor agonist, but this drug accomplishes a similar effect, albeit a little more extreme:
Edit: I don't think anyone should take it. I just mean that your dream could become reality if development on that drug continues.
No. Comparing to a control group is required, but it doesn't have to be a placebo control group. It's 100% acceptable, even required, to compare a new treatment to the standard-of-care treatment.
For example, if I were testing a new oral medication for Type 2 DM, I might randomize the participants to receive either the new med or metformin, which would act as the control. It would be unethical to randomize them to a placebo when they have a serious disease with a known effective treatment. This is my argument for GLP-1Ras, too.
It's tempting to ask for this, but it's not generally a good idea across the board.
When it comes to establishing efficacy (how well a drug works), a non-inferiority trial (what you're asking for) is suitable for that.
When it comes to establishing safety, it can be risky to allow for that. Here's a completely fictitious example:
Let's pretend Ozempic had a side effect where it deteriorated vision in 5% of people, while suffering from diabetes also had a 5% rate of vision deterioration. In conducting the original trial in diabetics, that safety signal would have been completely missed, since the people Ozempic saved from diabetes-related vision problems would now experience Ozempic-related vision problems. Perhaps those problems also take a couple years to show up. Approval is granted and nobody knows about that side effect, since just as many people in the control group as the active group had vision problems.
Next Wegovy (same active ingredient) is approved for weight loss (perhaps at a lower dose) based on a shorter trial in obese (but not diabetic people). Since it's a shorter trial length the vision problem isn't detected.
After that Zepbound is trialed (and we'll pretend it has that same side effect, but affects 8% of people). If Lilly ran Zepbound against a placebo then that risk would be discovered. Instead, Zepbound uses Wegovy (at its highest dose) as the control group. The small increase in vision problems isn't statistically significant and Zepbound is approved.
Next up is Retatrutide (let's say vision problems affect 10% of people). That would definitely be caught if the control group received a placebo, but since they receive Zepbound, it isn't noticed there either.
To be clear, I don't think any of these drugs cause vision problems. I'm just explaining why it's easy to miss safety signals in drug trials when the control group isn't receiving a placebo.
There's a new drug candidate called aleniglipron that is very similar to orforglipron, but just different enough not to step on Lilly's patent. (LOL)
Here's a blog post with a quick summary:
Structuring a better mousetrap, aleniglipron
It appears that it may be a contender. Good weight loss numbers in the early trials.
Here's a blog post with a quick summary:
Structuring a better mousetrap, aleniglipron
It appears that it may be a contender. Good weight loss numbers in the early trials.
Calm Logic
GLP-1 Specialist
Still tempting despite no COA.WWB has $195 for 12mg or $115 for 6mg so they seem to have the most competitive price.
Jack brown
GLP-1 Apprentice
If you search over on meso they have tested a lot of their products... can't find orfo but they may haveStill tempting despite no COA.
Calm Logic
GLP-1 Specialist
There isn't much love at Meso for orfo, even though no one anywhere has posted an actual experience. There is a theory there that you need big pharma's special excipients for sufficient bioavailability. I don't buy it. On some level, it actually makes me want to try orfo more to find out.
Last edited:
chmuse
Public Nuisance 🦜🐧🦅🦚🦃🦢🐓🦆🦉
Just throwing out there: anecdotal evidence seems to say something about the grey formulation just isn't working quite the same- probably because pills have so many variables (coatings effecting what part of the digestive tract it's released at, etc.) Obviously no really good studies. I bought a bottle from Uther, haven't tried it yet.
byefatlicia
GLP-1 Enthusiast
Please keep us posted on your experience🙂Just throwing out there: anecdotal evidence seems to say something about the grey formulation just isn't working quite the same- probably because pills have so many variables (coatings effecting what part of the digestive tract it's released at, etc.) Obviously no really good studies. I bought a bottle from Uther, haven't tried it yet.
Calm Logic
GLP-1 Specialist
Good reviews here for orfo by trial participants:
Calm Logic
GLP-1 Specialist
A domestic reseller I know on TG has some Uther left for $180 (100 pills, tested by Uther at a little over 9 mg each).
Could you tell me who. I think Uther is out and and I might want to stockpile. Also would love not to get involved with crypto for as long as possible.A domestic reseller I know on TG has some Uther left for $180 (100 pills, tested by Uther at a little over 9 mg each).
Calm Logic
GLP-1 Specialist
By the way, I had a good experience a while back with getting T30 from Uther's warehouse. But it's crazy the pricing is better from resellers than from Uther.
Last edited:
Calm Logic
GLP-1 Specialist
One example: R50 kit for $500 plus shipping (from Uther in China).
Resellers are the way to go for Uther, at least for GLPs. Uther gives good discounts it seems for bulk pricing, which are passed on by the resellers. Like in-stock R20 by Uther for $135 from PWGrove.
Resellers are the way to go for Uther, at least for GLPs. Uther gives good discounts it seems for bulk pricing, which are passed on by the resellers. Like in-stock R20 by Uther for $135 from PWGrove.
Last edited:
Calm Logic
GLP-1 Specialist

Exclusive: FDA leaders pushed to cut Lilly weight-loss pill review time
Leaders at the U.S. Food and Drug Administration have pressed internally for reviewers to speed up their evaluation of Eli Lilly's experimental weight‑loss pill, after the company pushed for a faster timeline, documents seen by Reuters show.
"[FDA] could decide on Lilly's pill as early as March 28 if a new timeline is adopted."
Self-pay cost to be $150 a month for lowest dose:

A New Form of GLP-1s Could Revolutionize Weight-Loss Treatment (Again)—And It's Available Now
They’re definitely easier and more affordable than injectables, but do they work as well? Here’s what you need to know.
Jellybelly54
GLP-1 Enthusiast
I would caution anyone researching orfor to keep and eye on your liver function studies especially if taking anything that is broken down by liver metabolism: statins, antidepressants , some cardiac medications, seizure meds, pain meds and many more. Oh, if you drink alcohol be very careful.
Most all prescription drugs have some sort of delivery system. Throwing some raw powder in a capsule and calling it a pill is like comparing a a pair of roller skates to a Mercedes sedan. They move you down the road but I would rather be in the Mercedes. It’s a lot safer! A lot of medications are very caustic to the distal esophagus especially if you have reflux. A proper designed pill or tablet will get it into the small intestine before being dissolved. These are just things to think about. No telling what Uther, SSa, peptide sciences and the likes are selling. I would love for someone to show a picture of the ORFOR pill that they are taking. I would bet money that it is a capsule. Maybe that is not an issue? We just don’t know yet so caution is advised.
Most all prescription drugs have some sort of delivery system. Throwing some raw powder in a capsule and calling it a pill is like comparing a a pair of roller skates to a Mercedes sedan. They move you down the road but I would rather be in the Mercedes. It’s a lot safer! A lot of medications are very caustic to the distal esophagus especially if you have reflux. A proper designed pill or tablet will get it into the small intestine before being dissolved. These are just things to think about. No telling what Uther, SSa, peptide sciences and the likes are selling. I would love for someone to show a picture of the ORFOR pill that they are taking. I would bet money that it is a capsule. Maybe that is not an issue? We just don’t know yet so caution is advised.
Trending Topics
Members Online
- yobculture
- ibembhy
- Anonyreta
- Dinogainsaurus
- beforetherush
- Occindemure
- Bobroberts41
- Bluemoon
- Labcat
- jiapolin2004
- Rp121
- alan wen
- HereForThePeptides
- fapsaurus
- GLP1Pharmacist
- MrBMG
- Manualtrt
- WeezinDaJuice
- Estarossa19
- Archibaldcrane
- GhostPepper
- DiDi1
- Kboo11
- Tupapibog
- threestack$$
- Fam66
- ambot88
- goatgod
- Sowilo
- GrouchyG
- squidis
- Aaron65
- LaBahia2
- Pepasp
- Slogger
- Ragnar
- fasting4life
- oldguy189
- kai1083
- Rich1054
- New2ton
- tete2026
- Ella HKfatcat
- Eyeinthesky
- yeddie
- J Arbey
- Silvurxlol
- One piece lover
Total: 279 (49 Members & 230 Guests)